Medical writing is a profession dedicated to communicating scientific and medical information to appropriate target audiences, such as healthcare professionals, regulators, patients, and the general public, to achieve certain objectives.
A medical writer, also called a medical communicator, is a professional who produces a range of scientific documents describing research results, product use, drug- or disease-related educational or promotional literature, publication articles, content for healthcare websites, health-related magazines, or news articles, and other medical information. Medical writers write scientific documents, marketing materials, and research papers covering recent breakthroughs, treatments, and drugs on behalf of clinical clients.
If you want to become a professional medical writer, you have landed on the right post. This blog gives you a quick and nice idea about the different types of medical writing, what this field focuses on, and which steps this process involves, in addition to discussing its different benefits that medical writers and the healthcare industry can enjoy.
Types of Medical Writing
In general, many people are unfamiliar with what medical writers do. As a matter of fact, many of them are unaware that medical writing is a career. In the pharmaceutical and healthcare communication industry, every medical writer generates different types of content. The following are the main and most common types of medical writing:
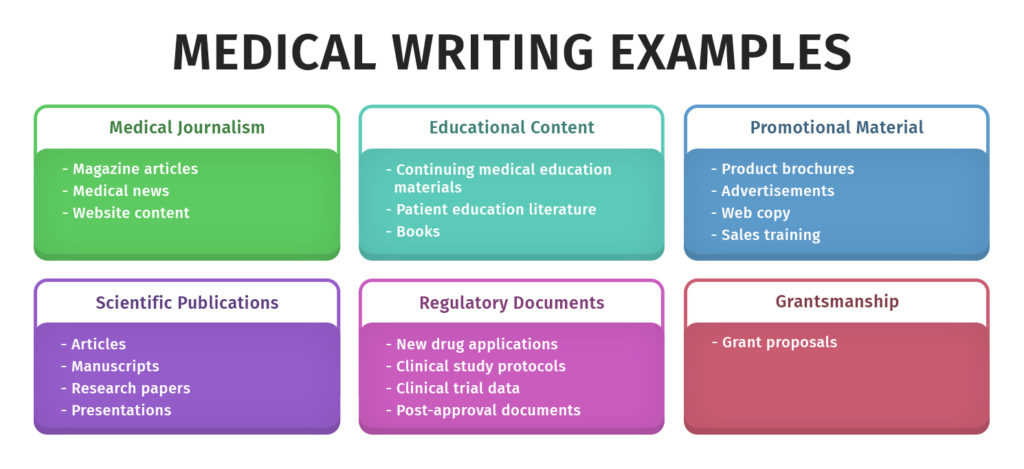
Medical Journalism
This type includes general medical news, featuring concise, journalistic-style stories that summarize the latest findings in clinical research. Medical writers write several news stories and attend medical conferences on a regular basis. In general, they write documents like newspaper and magazine articles for the general public. A writer who starts working as a medical news reporter can learn how to identify the most critical aspects of a research study, which are also helpful for other types of medical writing projects.
Medical Publications
Medical publication professionals work with physicians and researchers to write and edit posters, abstracts, manuscripts for refereed journals, and oral presentations for medical conferences. Medical writers are also medical journal editors who write and review journal articles. A medical communicator also creates summaries of conference sessions and news articles for professional audiences. Medical writers in the scientific publications area may work at educational or healthcare institutions or biotech or pharmaceutical companies.
Medical Education
Medical writing for educational purposes targets physicians, different healthcare experts, students, and patients with a particular medical concern. For physicians, pharmacists, and other medical professionals, many medical writers write textbooks, Continuing Medical Education (CME) programs, e-learning modules, literature review articles, slide decks, etc. Some medical communicators also write training materials for staff in biotech settings. They also write patient education material for students and patients, which falls under the medico-marketing writing category in pharmaceutical companies.
Medical Marketing for Healthcare Products
Medical writing for marketing purposes must have communicative and informative content. A professional medical writer writes content for pharmaceutical or biotechnological companies to meet their development and promotional purposes through the correct advertising information. They write and edit different materials for lay audiences, including product monographs, reviews, brochures, handouts, training manuals, advertisements, website content, etc.
Regulatory Documents
Regulatory medical writing is a form of technical writing that regulatory agencies need in the approval process for medical devices and drugs. It is the most formal type of medical writing, relying heavily on concise facts. Regulatory writers are essential to any pharmaceutical company. They write and edit documents for regulatory submissions, such as for marketing acceptance of new drugs or to perform new clinical trials. Some regulatory submission documents are Common Technical Documents (CTD), package inserts of drugs, subject narratives, annual reports, etc.
What Does Medical Writing Focus On?
If you think medical writing focuses on just a single thing, you are wrong. There is a lot happening in the medical and healthcare communication industry, and medical writers’ work focuses on various things. Here is what medical writing focuses on:
New Therapies and Drugs
Before testing any new medicine or therapy on human subjects, the drug developers apply for permission to run clinical studies by submitting an application. These applications summarize manufacturing information, scientific information available about the product, investigational plan, analyses to achieve study objectives, and information about the intention of the trial. Also, with the development program proceeding, the application needs to be updated for every new study. In addition, a number of key documents are needed to run the clinical studies. For all this, drug developers need medical writers to write and edit such documents.
Clinical Trials
During the clinical trials (research studies performed on people aiming to evaluate medical intervention), many documents need writing or updating throughout the course of a clinical program. Medical writers also work to make amendments to the CSPs that describe changes to the planned study or analyses, report drug safety data in the annual Development Safety Update Report (DSUR), and update the Investigator’s Brochure in preparation for the start of the upcoming studies. A competent medical writer also focuses on final statistical analyses of trial data and describes it in the Statistical Analysis Plan (SAP).
Medical Devices
Many companies seek competent writers to present information on novel medical devices. For implantable devices, for example, companies will have to provide a lay summary of the main safety and performance aspects of the device, with clinical evaluation outcomes, to the public. Medical writers use their scientific knowledge and writing skills to present such information or to write clinical evaluation reports (CER), clinical investigation plans, investigator brochures, and post-market follow-up studies.
Education and Research Results
New knowledge and information keep becoming part of the medical field through an ever-increasing number of research studies and novel ideas and thoughts. Medical writing focuses on communicating all this information in an effective manner to different audiences. Medical communicators collect research results and create various documents for developing educational activities for physicians and other practitioners. The field also focuses on creating educational material for students and patients having particular medical concerns.
What is the Medical Writing Process?
Medical writing enjoys great popularity in the medical industry. Writing comprehensive medical content has now become a great profession, which involves offering higher-level information to medical professionals and patients. To communicate scientific communication, there is a comprehensive medical writing process that a professional medical writer must follow. Here is the breakdown of the medical writing process:
Pick a Field
Choosing a medical field is of the utmost importance. Some notable fields for medical writing are medical education and training, health monitoring, and disease management. We advise you to choose a field that you understand, since a vague approach to any medical field can pose dangers. If you want to become a medical communicator, you must have substantial knowledge in all types of therapeutic areas. Medical writers need to be aware of the scientific regulations relevant to their subject and have sufficient expertise to produce comprehensive articles for doctors, scientists, specialists, regulators, patients, and non-medical readers.
Collate Research Data
Reading is important to collate relevant scientific information about a respective field of healthcare. You should learn about a chosen field and understand how it works. While doing this, medical writers should maintain the integrity of the research data and offer up-to-date medical information to the readers. Medical writers must have enough expertise to maintain complete accuracy and security of collected research data. Also, they must have enough skills to deliver information about the latest medical inventions.
Explore Relevant Medical Resources
The medical writing process also involves exploring countless resources. You can read peer-reviewed articles, medical books, and online resources to get help and gain knowledge about your chosen field of medical research. In doing so, however, you should follow relevant, authentic healthcare websites. All in all, medical writers should have a clear understanding of the process of exploring relevant resources that justify the type of article they are pursuing.
Write Lucid Medical Content
After collating data and exploring different resources, a medical writer writes scientific documents in the most lucid manner. As a medical writer, you must offer clear declarations of the objectives and organize ideas while adding information in a creative manner. You should use appropriate language and the level of technical information suited to the understanding of the respective audience. For this purpose, having an understanding of medical terms is important. While writing, you should understand the mindset of readers and offer an appropriate solution to the issue in a simple communicative manner.
The documents for regulatory submission must fulfill the defined structures and formats, and the content must follow the regulatory rules and other guidelines. The content should have a systematic arrangement and no spelling, punctuation, or grammatical mistakes. The main thing a medical writer should follow is the independent viewpoint and preparing the content within a decided timeline.
Get Acceptance
The last part of the medical writing process is the acceptance of the content by the medical professionals. It is ideal to get approval from all the stakeholders related to the chosen medical field. For that purpose, the content should have authentic medical information. To get approval from different audiences, medical writers share their written content on different community boards and medical conference pages. You can also enhance the scope of your medical journals by circulating them on social media.
What Are the Benefits of Effective Medical Writing?
To some medical professionals, writing is a source of enjoyment. The considerate healthcare experts write for the pleasure obtained from the creative writing activity, logical sharing, and the desire to increase knowledge and benefit the human race. For these authors, writing may act as a mode of expressing the pleasure of scientific discovery. But for others, the advantages they can gain from effective medical writing fall into the following categories:
- Career
- Professional
- Institutional
- Practical
Career Benefits
While training, the incorporation of written medical communication into the assessment and accreditation system recognizes the significance of written medical communication. A majority of medical specialty assessments entail a written component where candidates must write brief text within a short period of time. A good deal of international professional specialty qualifications include a mini-thesis, which demands elements of fundamental research techniques and manuscript preparation.
The major reason for many professionals to start medical writing is to meet the specific job requirements set by employers, i.e., medical institutions or universities. This involves the first appointment to an educational position, confirmation or renewal of that appointment, promotion to a higher-level position, and granting of tenure.
For some public and private hospitals worldwide, publication in recognized journals is a requirement for appointment as a consultant. It is also a must to have a first-author journal publication for specialty accreditation, as set by some specialist training committees. Another career benefit is the application for membership in reputable academic societies.
Professional Benefits
In general, the publication is a form of international currency that cuts across geographical boundaries. Having published articles in prestigious international journals helps young professionals a lot when they apply for positions in foreign centers and for competitive international fellowships. For established healthcare experts, publications are important to gain recognition as a professional in a certain field at national and international levels, leading to numerous benefits, such as appointments as consultants to government agencies, advisory and editorial boards, and expert panels, and invitations to lecture at scientific meetings.
Publications with a focus on a particular topic or field also help attain a certain standard of academic endeavor by various reputable global educational societies. From an educational perspective, writing and publishing can improve your prospects of succeeding in applications for research funding, funding extension, and obtaining further funding. All grant-awarding bodies also examine the publication track records of the investigators in detail.
Scientific study, research, and writing impose discipline, which, as a result, increases the professional’s depth of knowledge in a specific subject or field. This knowledge enhances and improves their clinical skills and enables better teaching of students. Through repeated scientific writing and publication in a certain field or topic, you, as a medical writer, can gain acknowledgment as a specialist by your peers.
Institutional Benefits
Publication in refereed articles is one of the most important means for an individual, department, university, or medical institution to achieve international recognition. In such cases, the author’s region and country may also receive an advantage from published work if it is on a particular major topic. Numerous educational organizations and government bodies use publications as a measure of academic productivity. You should know that published work contributes to an organization’s educational prestige and reputation and impacts the budget allocation for individual academic cost centers and departments.
Practical Benefits
The major practical reason for healthcare professionals to know how to write is the benefit got from the basic training gained during the manuscript preparation process. Medical writing involves the discipline of carrying out exhaustive literature research, collecting and analyzing data, and drafting and reviewing the manuscript over and over. Those authors with their manuscripts accepted and published will better appreciate what is in medical journals and other publications.
We recommend all healthcare professionals avail themselves of the opportunity to serve as journal manuscript reviewers. Having a great amount of information available in many journals and other scientific documents, it is important for medical healthcare experts and academics to have the ability to judge the quality and reliability of published work. Those who publish and appreciate the process of writing documents and editing them can better read and evaluate articles.
The ability to provide preferential assessment and judgment of the written content is the skill that will make you a better medical expert. After all, patients prefer to visit the most knowledgeable and informed professionals. So, looking up to and respecting the expertise of your knowledgeable peers will help you become better in your field.
The Bottom Line
The need for medical writers is growing in the pharmaceutical and healthcare industry since companies develop many new drugs and medical devices on a regular basis, and there is a demand for the generation of several scientific documents for submission to regulatory authorities during the approval process. The medical writing profession is capable of connecting scientific research results and medical interventions to both professionals and non-professionals. So, to become a part of this, all you need is to have knowledge in both writing and medical science, and combine your creative writing talent with the details of the scientific research process.
Frequently Asked Questions (FAQs)
Here are the most frequently asked questions about medical writing.
1. How much do medical writers typically earn?
Medical writer salaries vary based on experience, employer type, and job type. According to data from ZipRecruiter:
- Full-time medical writers in the U.S. typically earn between $70,000 and $150,000 per year.
- Freelance medical writers often charge an hourly rate between $50 and $150, or a project-based rate depending on the type of document and client.
Writers with specialized experience—such as in regulatory submissions or continuing medical education—can command higher rates, especially with a strong portfolio and proven track record.
2. What factors influence compensation in medical writing?
Several factors affect medical writing income, including:
- Experience level and years in the industry.
- Type of employer, such as pharmaceutical companies, medical communications agencies, or freelance contracts.
- Complexity and specialization of projects (e.g., regulatory vs. marketing).
- Quality and diversity of your portfolio and client base.
According to the AMWA Compensation Report, writers with advanced degrees (Ph.D., M.D., Pharm.D.) or expertise in medical devices tend to earn more, particularly when working independently or leading consulting firms.
3. What ethical standards guide professional medical writing?
Medical writers adhere to recognized ethics frameworks to ensure transparency and scientific integrity. Key references include:
4. How is medical writing used in digital and social media?
Medical writers are increasingly creating content for digital platforms—including social media, mobile apps, and video content—to communicate complex health topics in plain language. This involves balancing accuracy with accessibility for both healthcare professionals and the public. Writers must also adhere to best practices for tone, factual accuracy, and regulatory compliance, especially when discussing medical devices or public health issues.
5. What ethical challenges arise in digital medical communication?
In digital health communication, maintaining credibility and professional tone is vital. Writers must disclose sponsorships, avoid bias, and ensure compliance with advertising and privacy regulations. Upholding ethical principles helps combat misinformation, promotes transparency, and strengthens audience trust in health-related content across social media and digital channels.
6. What resources can help aspiring medical writers develop their careers?
To grow in the field, aspiring writers can use these top professional development resources:
- American Medical Writers Association (AMWA) – offers certification, networking, and continuing education.
- Council of Science Editors (CSE) – provides editorial and style guidance.
- AMA Manual of Style – the standard reference for scientific and medical communication.
- The Science Editor’s Bookshelf – a collection of foundational books on medical writing and publishing.
- Academic medical communication programs and continuing education courses for structured skill-building.
Developing skills in regulatory writing, medical reference research, and information synthesis helps writers stay competitive in this evolving profession.












